It’s no secret that batteries are holding back mobile technology. It’s nothing against the battery companies, which are surely dedicating quite a lot of R&D to improving their technology, hoping to be the first out of the gate with a vastly improved AA or rechargeable device battery. But battery density has been improving very slowly over the last few years, and advances have had to be in processor and display efficiency, in order to better use that limited store of power.
Researchers at Northwestern University claim to have created an improved lithium ion battery that not only would hold ten times as much energy, but would charge ten times as quickly.
It’s probably safe to call it a breakthrough.
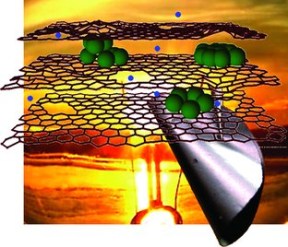
Inside Li-ion batteries, there are innumerable layers of graphene, a one-atom-thick sheet of carbon atoms. Lithium ions fill the spaces between these layers, and when the battery is being charged, these atoms must creep their way physically to the edge of the sheet in order to get down to the next layer and make room for more ions. The rate of recharge is limited by how fast these ions can go from layer to layer. One solution tried before was replacing the carbon sheets with silicon, which for some chemical reason can hold many times the lithium ions — but the silicon would expand and contract with the charge cycles, quickly breaking.
Professor Harold Kung, researcher at NU and lead author of the paper (published this month in the journal Advanced Energy Materials), has discovered not just one, but two techniques for improving this charge process. His lab decided to combine the strengths of both materials, carbon and silicon, by populating the area between the graphene sheets with silicon nanoclusters. These little clusters greatly increase the amount of ions that can be kept in the battery, and because they are small and the graphene is flexible, their size changes are manageable. Thus, the charge capacity of the battery was improved by, Kung says, a factor of ten.
But that’s not all. Kung’s lab also thought of perforating the graphene sheets, allowing ions to take a “shortcut” to the next layer. They call these 10-20nm holes “in-plane defects,” and they essentially rust them out. The result? Charging is ten times faster.
A possible downside is a faster degradation process; after 150 charges and discharges, the batteries showed only a 5x improvement to capacity and charge speed. Of course, those 150 charges would be the energy equivalent of 1500 charges of today’s batteries.
Naturally this huge leap in battery power and efficiency won’t be in your phones next week; they estimate they could be on the market in three to five years — cold comfort to iPhone 4S owners who are only getting seven or eight hours of on time. But the process is changed enough that existing manufacturing techniques are likely insufficient.
The full paper, In-Plane Vacancy-Enabled High-Power Si-Graphene Composite Electrode for Lithium-Ion Batteries, is available to subscribers here.