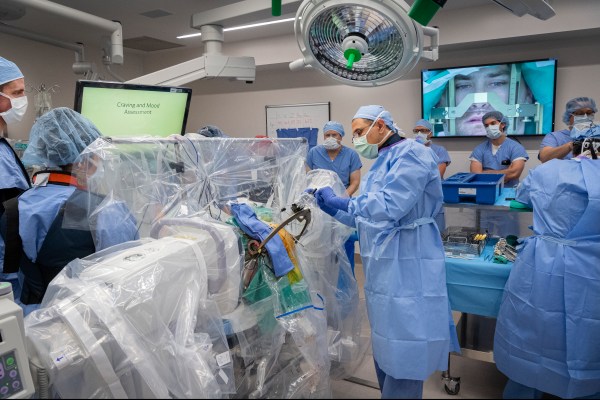
Opioid addiction is easily one of the top widespread healthcare issues facing the U.S., and research indicates we’re nowhere near achieving any kind of significant mitigating solution. But a team of medical researchers working at the West Virginia University Rockefeller Neuroscience Institute (RNI) and West Virginia University Medicine (WVU) are beginning a new clinical trial of a solution that uses brain-embedded technology to potentially curb opioid addiction in cases that have resisted other methods of treatment.
A team of neurosurgeons from RNI and WVU successfully implanted what’s called a “deep brain simulation” or DBS device into the brain of a 33-year-old man, the first person to participate in the trial. The DBS device consists of a number of tiny electrodes, attached to specific parts of the brain that are known to be associated with addiction and self-control behaviors. The DBS should, in theory, be able to curb addiction as related impulses are sent, and also monitor cravings in real time in the patient, providing valuable data to researchers about what’s occurring in cases of treatment-resistant opioid addiction.
Opioid addiction resulted in as many as 49.6 deaths per 100,000 people in West Virginia in 2017, WVU notes. That’s the highest rate of opioid-related deaths in the U.S. Other, less invasive treatment options are definitely available, including opioid alternatives that will provide pain relief to chronic sufferers like the one being developed by startup Coda. But for existing sufferers, and especially for the significant portion of opioid addiction patients for whom other treatments have not proven effective, a high-tech option like DBS might be the only viable course.
This RNI trial will initially include four participants, who have all undergone thorough courses of treatment across a number of programs and yet continue to suffer from addiction. The team involved also has extensive experience working with DBS in FDA-approved treatment of other disorders, including epilepsy and obsessive-compulsive disorder. It’s definitely a last-resort approach, but if this clinical trial produces positive results, it could be an option to help the most serious of cases when all other options are exhausted.