For the past 19 years, Ioannis Tarnanas, the founder and chief scientific officer at Altoida, has been developing virtual and augmented reality tools to offer predictions about the onset of mental illness in older patients.
The company, whose tools have been cleared by the Food and Drug Administration (an earlier version of this article incorrectly stated the technology was approved) for predicting Alzheimer’s by measuring brain function, claims that it can determine with a 94% accuracy whether someone will present with the disease six to 10 years before the onset of mild cognitive impairment symptoms.
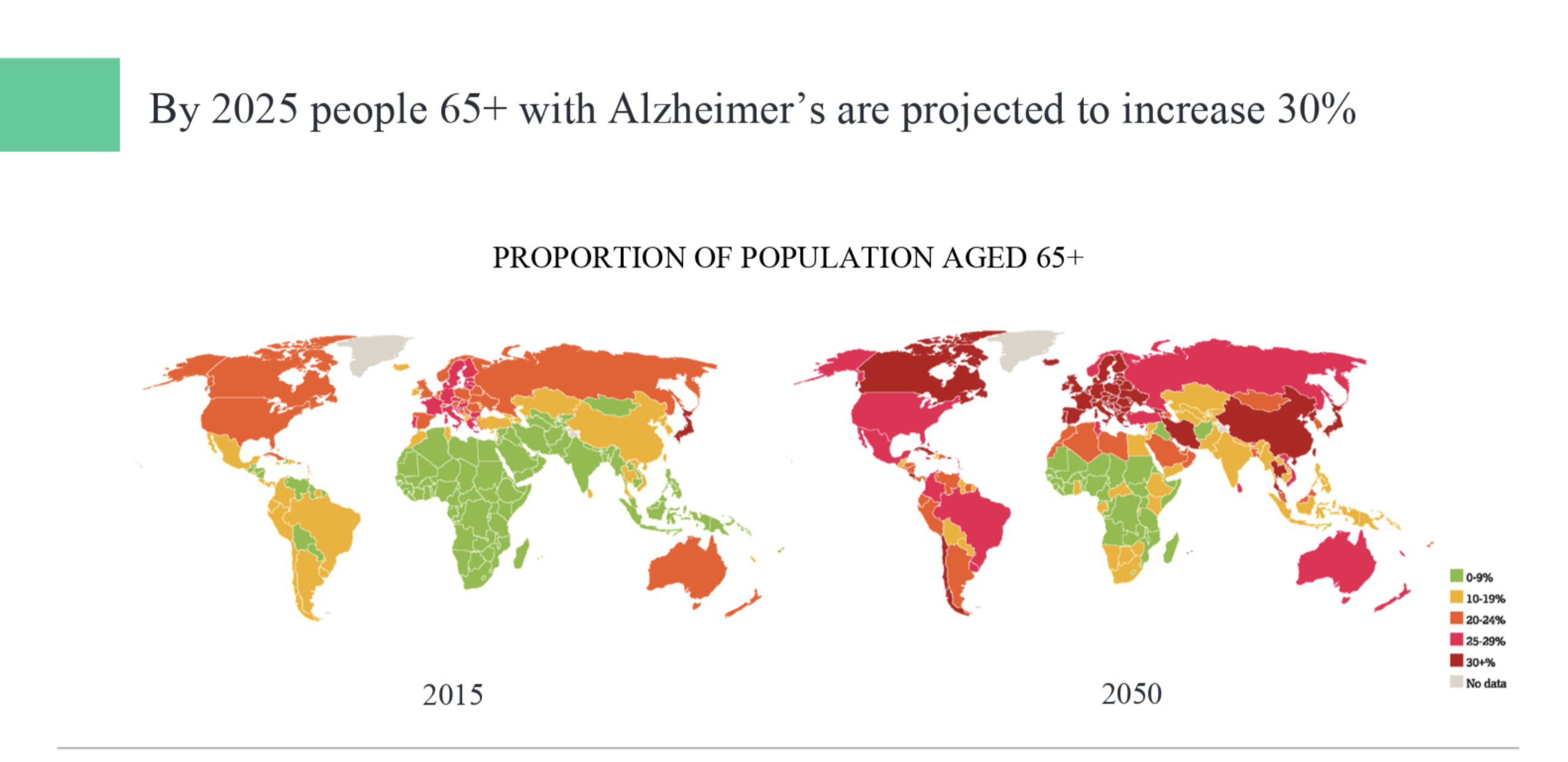
In 2019, Alzheimer’s and other dementias will cost the U.S. nearly $290 billion, and that figure could rise as high as $1.1 trillion by 2050, according to Altoida.
The number of people living with Alzheimer’s disease is rapidly growing. In 2019 alone, Alzheimer’s disease and other dementias will cost the nation $290 billion. By 2050, these costs could rise as high as $1.1 trillion, but Altoida says that these costs can be prevented if the disease is caught early enough.
Altoida uses an iPad or a tablet accelerometer, a gyroscope and touch screen sensors to detect what the company calls “micro-errors” as patients complete a series of AR and VR challenges. It’s basically a game of hide-and-seek where patients put virtual objects in different physical spaces in a clinical environment and then try to collect them.
Right now, the company’s technology is only available as a clinically supervised test in a doctor’s office, but the company is beginning to look at bringing its diagnostic tools into the home.
“In this field there are two major waves. Passive digital biomarkers and active digital biomarkers. With passive biomarkers you collect data from sensors,” says Tarnanas. “To give you an example of what this means in real life, [with passive digital biomarkers] you wind up collecting huge amounts of data and you see spikes and associate that with more everyday function or not… you are never sure whether this is due to day to day activity.”
Tarnanas started conducting longitudinal clinical trials around cognitive testing in the early 2000s while he was working on his Masters at the University of Sussex. He then moved to San Diego and worked in the Virtual Reality Medical Center before moving on to Bern, Switzerland to conduct additional research. Tarnanas finally settled in Houston, where Altoida is now based.
“Developing enhanced methods to objectively evaluate cognitive function is a critical component of the next generation digital medicine — a component that is required to not only advance the basic research in neurodegenerative disease, but also one that is required for the development of improved clinical interventions,” said Dr. Walter Greenleaf, PhD, a neuroscientist and Distinguished Visiting Scholar working at the Stanford University Virtual Human Interaction Lab, in a statement. “Understanding neurodegenerative biotypes will dramatically improve our ability to conduct a differential diagnosis at the primary care level. Improved diagnostics will provide healthcare professionals with the key information necessary to precisely adapt clinical interventions to personalize the patient’s cognitive care. This will ultimately lead to improved outcomes of care and to reduced healthcare costs.”
Some influential healthcare investors are already on board. Altoida has raised $6.3 million in a new round of financing from investors led by M Ventures, the corporate investment arm of the pharmaceutical company Merck, with additional participation from Grey Sky Venture Partners, VI Partners AG, Alpana Ventures and FYRFLY Venture Partners.
“The beauty of active digital biomarkers is that they can actually expand to more conditions,” says Tarnanas. The company is looking at expanding its prognostic toolkits to determining lasting impacts from traumatic brain injuries, and post-operative cognitive disorder, he says.
“As the world’s effort to introduce meaningful therapies for Alzheimer’s disease inches closer and closer to success, it is clear that the greatest benefit will come to those whose disease is detected at a very early stage,” said Jonathan L. Liss, MD, director at Columbus Memory Center and Founder of Columbus Memory Project, who has been using Altoida’s technology since September 2018. “The Altoida Neuro-Motor Index (NMI) device offers an ingenious way in which to detect early disease and track progression without prolonged cognitive testing, tissue sampling or radiologic intervention. The Altoida NMI device is a welcome advancement to the field of cognitive health.”
Altoida isn’t alone in trying to find a way to diagnose Alzheimer’s earlier. Recently, MyndYou, a New York-based company, announced a partnership with Mizuho to bring its passive prognostic toolkit to Japan. That company recently secured roughly $2 million to build out its own solution.