The Food and Drug Administration has just cleared AliveCor’s Kardiaband EKG reader as the first medical device accessory for the Apple Watch.
Europe has been able to use a version of the Kardiaband for Apple Watch for some time now but, thanks to the new FDA approval, the device can now be used in the U.S., marking the first time an Apple Watch accessory will be able to be used as a medical device in the States.
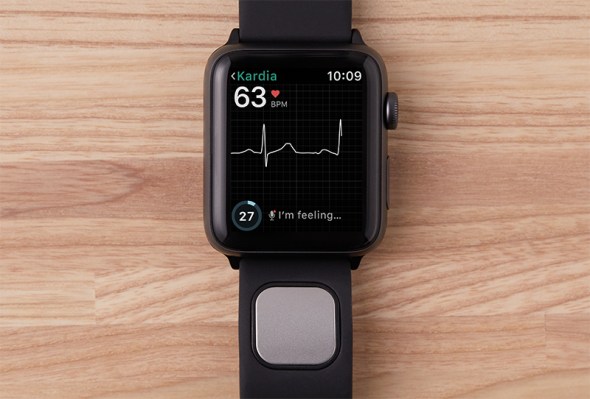
Up until now, AliveCor has used the KardiaMobile device, which was stuck to the back of your smartphone and paired with an app to detect abnormal heart rhythm and atrial fibrillation (AFib). The new Apple Watch accessory, Kardiaband, clicks into a slot on the Watch band to do the same thing.
However, rather than needing to hold your smartphone with both hands for 30 seconds to get a reading, you can get an EKG reading continuously and discreetly just by touching the band’s integrated sensor.

Along with the new Kardiaband for Apple Watch announcement, AliveCor is introducing a software feature called SmartRhythm, which uses a deep neural network to give you insight into your heart rate and can potentially detect an abnormal heart beat using the Kardiaband or KardiaMobile EKG reader.
Note, there have been a couple studies conducted using just the Apple Watch’s built-in heart rate monitor to detect an abnormal heart rhythm. This spring, UCSF and Cardiogram conducted one such study, concluding the Apple Watch could detect an abnormal heart rhythm with a 97 percent accuracy when paired with an AI-based algorithm called DeepHeart.
Later, the same eHealth study concluded the Watch could also detect sleep apnea and hypertension with similar accuracy using its built-in sensor.
But, as AliveCor CEO Vic Gundotra points out, it’s one thing to be able to detect and another thing to get FDA approval to use your sensor as a medical device.
“Apple might be able to say ‘oh your heart rate is high’ …but what does that mean? Does that mean you should go to the hospital? And if you go to the hospital what are they going to do?. Any doctor will say ‘ok come in, let’s get an EKG reading’,” Gundotra told TechCrunch.
EKGs are usually only available in offices and hospitals — and only after a life-threatening event. Having one on your wrist that you can use to check your heart and then send a readout straight to your doctor is vital to prevention of a heart attack or stroke.
And, as Gundotra also points out, “It’s not possible to diagnose atrial fibrillation without FDA clearance. That is a big, big play.”
It’s worth noting Apple could easily replicate what AliveCor is doing. It has all the right equipment within the Apple Watch and the manpower to do so. However, it doesn’t seem likely Apple would want to go through the hassle of FDA approval for the Watch, which is a general purpose device used for numerous other applications besides getting your heart rate.
The FDA has also told TechCrunch in the past that it would be the software, not the platform on which it operates, that would be regulated anyway.
That’s not to say someone else couldn’t come up with an FDA-approved EKG reader but so far AliveCor seems to have the market on that for both the KardiaMobile and now the Kardiaband.
That’s an important marker for the company. AFib is the most common heart arrhythmia, and a leading cause of stroke. In fact, one in four adults over the age of 40 could be at risk.
“This is a medical device. This is not a toy that says your heart might be irregular. This is an FDA-cleared device. It’s one of the hardest things I’ve ever done in my life,” Gundotra said.
Those interested can get their own Kardiaband starting today for $199 on AlivCor’s site. The band does require a subscription to AliveCor’s premium service for $99 a year.