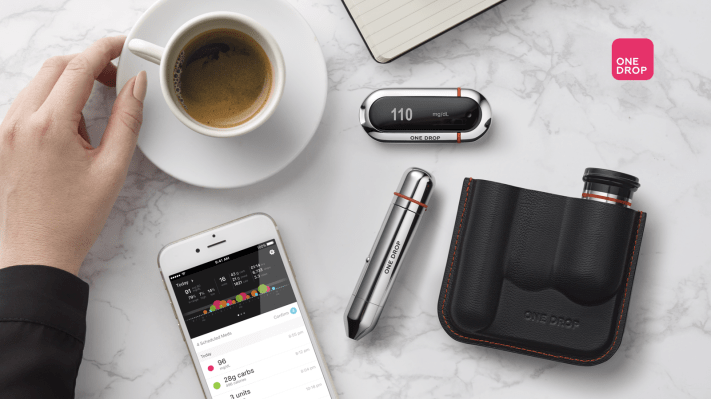
Last summer, One Drop raised $8 million in Series A funding for its diabetes management app. At the time, the company said the money would go, in part, to developing hardware designed specifically for its mobile health software.
The result is the Chrome, a glucose monitoring system that, well, looks pretty slick, so far as glucose monitoring systems go. It’s a four-piece set that includes standard test strips, a lancing device, Lancets and a connected Bluetooth meter. The system has been given the go-ahead from both the FDA in the states and received the CE marking in Europe (in record time, according to the company).
As such, it’s set to go on sale this month through One Drop’s site and the Apple Store here in the States. It’s a fitting exclusive, given Apple’s recent focus on health hardware and software over the last few years.
The Chrome is coupled with a new monthly subscription service from the startup that promises both unlimited test strips and 24/7 access to certified diabetes educators by way of the company’s mobile app. The non-premium version also gives users access to glucose and medication data, syncing up fitness info from other apps.