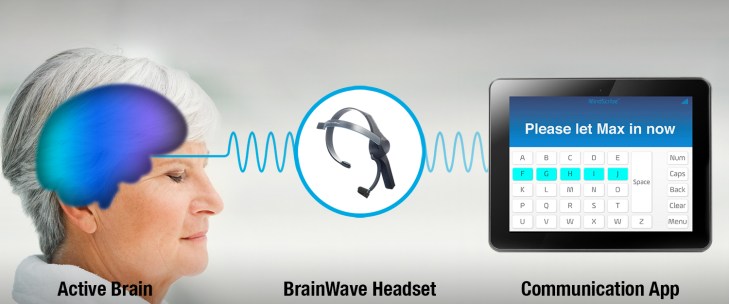
NeuroSky, the company that brought you the technology behind the Star Wars “Force Trainer,” is moving farther away from the dark side with its development of the MindScribe headset and accompanying software.
The MindScribe, which interprets brain waves, is designed for people suffering from what is called “locked-in syndrome.” Locked-in syndrome is the inability to communicate that results from damage to the brain-stem. Patients suffering from this condition retain consciousness, but are unable to verbally express themselves.
The device will allow for three different modes of communication. The first will be an expression mode to identify and share emotional state. The software will also come with a yes/no response mode and a typing mode.
One of the biggest obstacles for patients suffering from locked-in syndrome is the immense costs that arise from treatment. Many insurance providers do not cover emerging-technologies that assists patients in communicating.
Typically NeuroSky focuses its resources on building and selling sensors to other manufacturers who integrate the technology that detects the electrical activity of the brain. But by vertically integrating the hardware and software components, NeuroSky hopes to bring down prices significantly.
The initial rollout of the MindScribe headset will occur next month on Indiegogo. This might seem like a strange move for a technology that has already been developed, but NeuroSky wants to use the Indiegogo for publicity. The first 1,000 customers will be able to get the MindScribe headset and software for $500. Eventually the price will return to around $1,500.
Traditionally, patients suffering from locked-in syndrome would have custom devices built to take advantage of any movement ability they retained. A patient could spend upwards of $15,000 on a device that could register only toe or eye-lid movement.
Because the device is not taking any medical readings and is solely for communication, the company doesn’t need to attain FDA approval. The time-consuming FDA approval process can drive venture-backed founders out of the medical device space. A number of companies are developing brainwave technology, but most have focused their efforts on commercialization in consumer spaces like gaming and VR.
Because the goal for the endeavor is to breakeven and not hit 10X, NeuroSky might be able to pull it off. In the event the MindScribe does generate a profit, NeuroSky wants to give a percentage to the ALS association to support research and support for patients with locked-in syndrome.