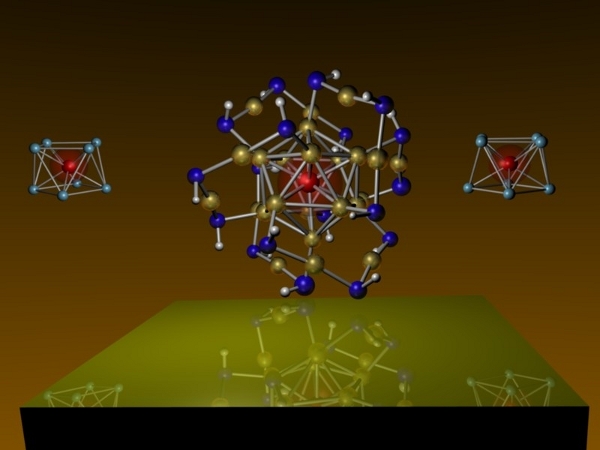
Scientists at Virginia Commonwealth University have revealed in the journal Nature Chemistry that they’ve created a “magnetic superatom”, or “a stable cluster of atoms that can mimic different elements of the periodic table”. The poor Periodic Table of the Elements barely gets any respect as it is from the kids today (quick: do you know your Noble Gases, or the atomic weight of Nobilium?), now it has to compete with fancypants super atoms!
This new superatom is made of one vanadium and eight cesium atoms, and it “acts like a tiny magnet that can mimic a single manganese atom in magnetic strength while preferentially allowing electrons of specific spin orientation to flow through the surrounding shell of cesium atoms”. What good is this new superatom? According to Shiv N. Khanna, Ph.D., professor in the VCU Department of Physics:
A combination such as the one we have created here can lead to significant developments in the area of “molecular electronics,” a field where researchers study electric currents through small molecules. These molecular devices are expected to help make non-volatile data storage, denser integrated devices, higher data processing and other benefits.
The researchers are also exploring non-conductive magnetic superatoms by combining gold and manganese.
Via Spintronics Info.